When pain researchers think about ion channels and mechanical pain, which specific channels first come to mind? PIEZO2 may sit near the top of the list for many investigators, perhaps followed by the TRP channels TRPA1 and TRPV4, all of which have been implicated in touch-evoked pain. But if a new study is any guide, it's time for the less-heralded TRPC5 to step into the limelight.
The study, led by Cheryl Stucky, Medical College of Wisconsin, Milwaukee, US, shows that TRPC5 regulates mechanical pain, as well as spontaneous pain, in a wide variety of pain models in mice. TRPC5 does so via lysophosphatidylcholine (LPC), a phospholipid upregulated in the pain models, and that serves as an endogenous agonist of the channel. Demonstrating the translational potential of their work, the team also documents high expression levels of TRPC5 in human dorsal root ganglion neurons and provides evidence that LPC can activate human TRPC5.
“This is a significant paper. It really puts TRPC5 into the landscape of pain, going into more detail than previous studies and giving it a more significant role,” said Angelika Lampert, a pain researcher at RWTH Aachen University, Germany. “The paper stands out because it's using so many different models, and in the end the authors even try to translate the animal work to the human system, which is very important,” according to Lampert, who was not involved with the new research.
The research comes hot on the heels of another recent paper showing a role for TRPC5 in cold pain in teeth (see related PRF news article), and appeared May 26, 2021, in Science Translational Medicine.
Consistency across pain models
The new study had its beginnings when Kate Sadler, co-first author along with Francie Moehring, was in the first year of her postdoc in Stucky's lab and working with a mouse model of sickle cell disease (SCD). Previous work had found dysregulated circulating phospholipids, both in SCD patients and in SCD animal models. One of those phospholipids, LPC, was highly upregulated in sickle cell mice, as the authors would verify in their new study. There was also previous evidence suggesting LPC could act as a TRPC5 agonist.
“Then it sort of snowballed into, let's test every single model that we have in Cheryl's lab and see if this thing that we originally identified in our sickle cell model could be broadly applicable to all of these other interesting, persistent pain models that we have in the lab,” Sadler told PRF.
So the group obtained global TRPC5 knockout mice and observed the effects that this experimental manipulation had in a battery of pain models. First, wild-type control mice that received an intraplantar injection of complete Freund's adjuvant (CFA), an inflammatory pain model, showed mechanical allodynia two hours after injection, as did the TRPC5 knockout mice. But only the knockouts improved over time, exhibiting less allodynia a day after injection and no allodynia at all two days after injection, whereas the wild-type animals had allodynia for more than 40 days. Nor did the knockouts exhibit hypersensitivity to noxious punctate (needle) hindpaw stimulation or dynamic mechanical allodynia (in response to paintbrush swiping) several days following CFA injection. Meanwhile, a TRPC5 small molecule inhibitor injected into the paw eliminated mechanical allodynia in CFA wild-type animals, further implicating the channel in that model.
“The behavioral phenotype of the CFA animals is really striking and shows that TRPC5 is clearly involved in producing the painful response. It will be really exciting to learn from future studies in which cell types, sensory and non-sensory, TRPC5 is functioning and what signaling pathways are involved,” wrote Alexander Chesler, National Center for Complementary and Integrative Health, National Institutes of Health (NIH), Bethesda, US, in an email to PRF; Chesler was not involved with the new study.
The group then saw remarkably similar effects across other pain models, including a hindpaw incision model of postsurgical pain, a chemotherapy (paclitaxel)-induced model of peripheral neuropathy, and the authors' sickle cell model. Though not included in the current study data, findings from the sickle cell model when the investigators used a significantly higher dose of TRPC5 inhibitor were particularly compelling to the group that the channel was the culprit.
“We observed a complete reversal of all mechanical hypersensitivity in our sickle cell mice; typically, these mice are chronically super responsive to mechanical, cold, and heat stimuli. When I got that first result, I was like, holy smokes, this is definitely real. And then we had other people in the lab do the exact same experiment and see something very similar. That to me was the most convincing part,” Sadler said, along with the reversal of hypersensitivity seen in the other models reported in the new study.
The only exception to the findings was in a spared nerve injury model of neuropathic pain, in which knockout of TRPC5 had no effects on measures of mechanical hypersensitivity. This could be because serum and nerve levels of LPC were unchanged in that instance.
Importantly, the group also found that TRPC5 inhibition had no effect on pain or touch sensation in uninjured mice.
“The fact that inhibition of TRPC5 reverses the mechanical pain in all these injury models but doesn't do anything to inhibit baseline touch sensitivity is really, really critical,” said Stucky, saying this is in contrast to the behavior of PIEZO2, another channel linked to mechanical pain that has received much recent attention. “We never found with either the global knockout or with the compounds we were injecting that TRPC5 blockade dulled tactile sensitivity, leading TRPC5 inhibitors to be a really strong therapeutic for mechanical pain,” Stucky continued.
The researchers also emphasized the temporal aspect of their findings, where blocking TRPC5 had no effect immediately after injury, but only after injury had already been established. This suggests to them that other mechanically sensitive channels play a role early after injury, with TRPC5 coming into play later on.
TRPC5: Where is it? What is it doing? How is it playing a role in pain?
After observing the beneficial effects of the TRPC5 inhibitors, the group next turned its attention to the channel itself, aiming to identify which dorsal root ganglion (DRG) neurons expressed it. Using RNAscope, an in situ hybridization approach with single-cell resolution, to identify messenger RNA expression, the investigators saw expression of Trpc5 in 15% of DRG neurons from uninjured mice. The channel was expressed mainly in non-peptidergic C-fiber neurons as well as in A-delta and A-beta fiber neurons.
That's where TRPC5 was – but was it contributing to hypersensitivity in the animal models? Ex vivo electrophysiological recordings of tibial C-fibers revealed increased mechanical thresholds in TRPC5 knockout animals that had been injected with CFA, compared to fibers from CFA-injected wild-type mice. Afferents from the knockouts also showed reduced firing in response to application of force to the skin.
The researchers then suspected that a chemical mediator in injured tissue might be sensitizing or activating TRPC5 in the CFA model. Mass spectrometry results pointed to LPC, which was notably elevated in skin, but not serum, from wild-type and knockout animals just two hours after receiving CFA as well as a week after CFA injection. Results from the incision model and paclitaxel model were consistent with that finding. Meanwhile, there were no changes in sciatic nerve or serum LPC concentrations in the spared nerve injury model.
The next step was to understand the potential contribution of LPC to pain. So the group gave hindpaw injections of LPC to wild-type and TRPC5 knockout mice. While the wild-type animals developed mechanical allodynia, this was not observed in the knockout mice.
Then, extending their work to yet another pain model in collaboration with Gregory Dussor, University of Texas at Dallas, US – in this case, a migraine model where substances are injected onto the dura mater – the researchers found that, in wild-type mice, dural LPC increased mechanical sensitivity around the orbit of the eyes. But this effect did not occur when LPC was administered along with a TRPC5 inhibitor. Whole-cell patch-clamp recordings of trigeminal neurons exposed to LPC revealed that the phospholipid sensitized neurons in wild-type animals but not in the knockouts.
“I very much like that the group is showing this regulation by LPC,” said Lampert. “I think LPC is a new regulatory factor that could also potentially be interesting to consider for other ion channels that could be regulated by this metabolite.”
Where is the LPC coming from? The researchers believe that the answer to that question likely depends on the pain model – sickled red blood cells in the SCD model, perhaps immune cells recruited to the site of injury in inflammatory pain models, and maybe keratinocytes in the skin incision model. “Any time a cell membrane is lysed, you could get release of LPC into the local interstitial fluid,” Sadler said.
Spontaneous pain
The team also tested the effects of LPC on persistent spontaneous pain, a problem in many patients who suffer a nerve or inflammatory injury. Calcium imaging experiments in human embryonic kidney (HEK) cells transfected with mouse TRPC5 showed that LPC could activate the channel, with several different TRPC5 inhibitors each able to decrease the calcium flux that LPC caused.
Next, according to conditioned place aversion experiments, wild-type animals developed an aversion to a chamber that had been paired with subcutaneous LPC, but TRPC5 knockout animals did not. Further, wild-type mice that had been treated with paclitaxel preferred a chamber that had been paired with a TRPC5 inhibitor, whereas vehicle-treated controls showed no such preference. Together, the results pointed to a role for LPC, working through TRPC5, in spontaneous pain.
A number of further experiments showed that even after removal of extracellular LPC, mechanical hypersensitivity mediated by TRPC5 persisted. For instance, electrophysiology work showed that trigeminal neuron sensitization in response to LPC persisted 48 hours after in vivo application of LPC in wild-type but not knockout animals. Consistent with those results, in a behavioral model of migraine, infusion of neutral (pH 7.0) synthetic interstitial fluid onto the dura caused facial mechanical allodynia in wild-type animals that had previously received a dural LPC infusion but whose mechanical thresholds had already recovered – all this in the absence of LPC – but this behavior was not seen in TRPC5 knockouts.
Finally, additional work in a transgenic SCD model supported the findings from the other models. For instance, SCD mice, which have higher circulating LPC concentrations than controls, showed reduced mechanical allodynia as well as reduced noxious mechanical hypersensitivity upon receiving a TRPC5 inhibitor. But the inhibitor still managed to decrease the mechanical sensitivity of isolated DRG neurons from the SCD mice when LPC was no longer around. This result, along with some additional electrophysiology, suggested that TRPC5 keeps wreaking havoc even when LPC isn't around anymore.
Taking it into people
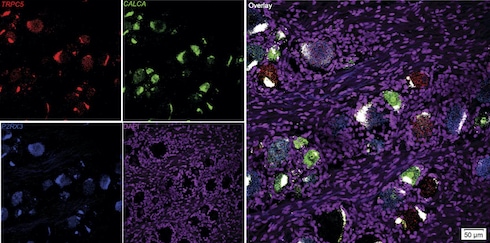
Pain researchers increasingly recognize that experiments on human tissue are needed to understand whether what holds true in the pain system of rodents applies to people. As big believers in that approach, the authors were able to obtain human DRG from tissue donors by collaborating with Ted Price, University of Texas at Dallas, US. RNAscope experiments showed that a whopping 75% of DRG neurons from the donors expressed TRPC5, especially medium- and small-diameter neurons.
“We were just blown away by the number of human DRG neurons that express TRPC5,” Stucky told PRF.
Most of the TRPC5+ neurons were nociceptors, co-expressing either the nociceptive marker CGRP or P2X3R, with a small percentage likely A-beta mechanoreceptors. Meanwhile, LPC could activate human TRPC5 channels expressed heterologously in HEK cells, and this activity was blocked by TRPC5 inhibitors. In short, as in mice, as in people.
The researchers are particularly excited about the translational potential of their findings, considering that two Phase 1 clinical trials of TRPC5 inhibitors have already been completed, one for kidney disease and one for affective disorders, and are now entering Phase 2.
“One attractive thing about targeting TRPC5 is that those trains have already left the station and they're in progress,” said Stucky, referring to the clinical trials.
That the inhibitors are being developed for affective conditions should be particularly intriguing to the pain field, considering that pain has affective aspects; perhaps a TRPC5 inhibitor could provide a win-win by treating both affective and sensory/discriminative components of pain.
In the meantime, there are many future research avenues to pursue. Lampert said that functional studies on human cells, such as human DRGs or induced pluripotent stem cell-derived nociceptors, are in order. Here, researchers could study the effects on those cells of applying LPC and of blocking TRPC5. Lampert also said that how LPC affects mechano-transduction mediated by other ion channels, and what the effects of conditional TRPC5 knockout specifically in the nociceptive system may be, are also intriguing questions to go after.
As for the study authors, one question of particular interest to Sadler, considering her focus on the amygdala during graduate school, is to assess the activity of TRPC5 in that brain structure, where the channel is highly expressed, and learn how TRPC5 might be contributing there to pain and co-morbid affective disorders.
“The fact that Kate is going into the CNS areas with TRPC5 is super exciting,” said Stucky, who further noted that the channel is also highly expressed in other brain areas that may come into play during chronic pain. Looking at patients with pain and what tissue levels and function of TRPC5 they have is yet another research path.
Finally, the group is also highly intrigued to follow up on the role of TRPC5 in migraine. A recent paper (Kuburas et al., 2021) reported that most mice in the study showed aversion to light after an infusion of pituitary adenylate cyclase-activating polypeptide (PACAP), a migraine mediator, but some of the animals did not. In animals that did respond to PACAP, TRPC5 was upregulated within the trigeminal ganglia.
“There is absolutely something to go after with regard to peptide signaling, migraine, and TRPC5. Could TRPC5 be a convergent point at which CGRP [another well-known migraine mediator] and PACAP meet at the trigeminal ganglion to drive migraine-like pain in animals, and even migraine in people? That's a really interesting idea,” Sadler said.
Neil Andrews is a science journalist and executive editor of PRF.