It is a cruel irony that the same drugs used to treat migraine headache can themselves cause headache. But the underlying causes of this phenomenon, known as medication-overuse headache (MOH), are unclear. A recent mouse study identifies a new mechanism responsible for MOH, focusing on a familiar suspect in pain.
Researchers led by Patrick Delmas, Aix-Marseille-Université, CNRS, Marseille, France, set their sights on Nav1.9, a voltage-gated sodium channel recognized for its role in pain. Using a mouse model of MOH induced by the anti-migraine drug sumatriptan, along with Nav1.9 knockout mice, the team found that nitric oxide (NO), a well-known migraine trigger in people, maladaptively activated Nav1.9 in dural afferent neurons. This led to increased excitability of meningeal nociceptors and headache symptoms.
The researchers also showed that activation of Nav1.9 caused release of calcitonin gene-related peptide (CGRP), dilation of meningeal blood vessels, and mast cell degranulation, resulting in the release of inflammatory substances that further potentiated Nav1.9 activity.
“Adding information to the big puzzle of MOH, and migraine more broadly, is essential for scientists to find novel targets for the development of new therapeutics,” said Milena De Felice, University of Sheffield, UK. “Additionally, the authors’ development of a MOH model in mice offers the possibility to investigate more and more specific targets using genetically modified mice,” according to De Felice, who studies migraine using preclinical models but was not involved with the current work.
The study was published September 18, 2019, in Nature Communications.
Nav1.9 is present and functional in trigeminal neurons
Previous studies had revealed that Nav1.9 plays a role in inflammatory and neuropathic pain in animal models, and that variations in Scn11a, the gene that encodes Nav1.9, lead to congenital insensitivity to pain as well as painful conditions in people. To learn more about the channel’s role in MOH, the authors looked at trigeminal ganglion neurons, which relay information from primary afferent fibers to the meningeal vasculature.
“We knew from previous research that MOH was linked to the sensitization of the trigeminovascular system,” said Delmas. “We decided to investigate the mechanisms of trigeminal excitability with a special focus on the role of Nav1.9.”
To begin, the researchers, including first author Caroline Bonnet, performed immunostaining of mouse trigeminal cryosections to document the presence of Nav1.9. They saw that the channel was expressed in about one-third of labeled trigeminal neurons. In addition, about 90 percent of meningeal fibers showed expression of Nav1.9, and a similar number expressed CGRP receptors, suggesting co-distribution of both throughout those fibers. Patch clamp recordings, as well as genetic activity assessments, revealed that about half of dural afferent neurons expressed functional Nav1.9 channels.
A role for Nav1.9 in a mouse MOH model
To examine if Nav1.9 contributed to MOH, the authors first needed an animal model of MOH. So they deleted the Scna11 gene in mice to create Nav1.9 knockouts. They then gave sumatriptan, a selective serotonin receptor agonist, to the knockouts and their wild-type littermates for six days.
Sumatriptan-treated wild-type mice showed decreased hindpaw mechanical withdrawal thresholds, compared to saline-treated wild-type animals. About three weeks after sumatriptan was first started, mechanical thresholds in the wild-type mice returned to pre-sumatriptan levels, and the researchers then administered sodium nitroprusside (SNP), a nitric oxide donor.
“Mice behave similarly to people and become extra-sensitive to migraine triggers when treated chronically with triptans,” Delmas said.
Indeed, SNP heightened allodynia, measured by paw withdrawal thresholds to von Frey hairs, in wild-type mice that had received sumatriptan. But this effect was absent in Nav1.9 knockout mice that had received sumatriptan. This showed an important role for the channel in SNP-induced allodynia in sumatriptan-treated wild-type mice.
Further, while all of the above experiments were performed in male animals, additional work in females revealed similar findings. Finally, the researchers also saw that, similar to paw allodynia, periorbital allodynia was greater in wild-type animals that had received sumatriptan compared to the drug-treated Nav1.9 knockouts.
The disabling symptoms of MOH commonly include increased sensitivity to light (photophobia) and sound (phonophobia). So the authors next investigated aversive behavioral responses to light and sound in the Nav1.9 knockout and wild-type mice.
Sumatriptan-treated wild-type mice displayed increased sensitivity to light, measured by a light-dark transition test in which the animals spent more time on the dark side of a chamber than on the light side, compared to saline controls. But upon SNP injection, sumatriptan-treated Nav1.9 knockout mice behaved similarly to saline-treated wild-type and to saline-treated knockout mice, spending no more time on the dark side compared to these control animals. Nor did the sumatriptan-treated knockouts show signs of phonophobia.
Together, the above experiments showed that Nav1.9 plays a key role in the development of NO-induced symptoms of central sensitization in the mouse MOH model.
Abnormal Nav1.9 activation leads to CGRP release, vasodilation, and mast cell degranulation
A number of electrophysiological experiments would show increased activation of Nav1.9 by NO, along with increased action potential firing, in dural afferent neurons from wild-type animals treated with sumatriptan, but not in the knockouts. The authors turned their attention to the downstream consequences of this.
They saw that activation of Nav1.9 with SNP enhanced the secretion of CGRP in trigeminal cultures from sumatriptan-treated wild-type mice, but this was not seen in cultures from Nav1.9 knockouts that received sumatriptan. Treatment with an intravenous CGRP antagonist decreased mechanical allodynia in response to SNP only in wild-type mice that had received sumatriptan and not in the sumatriptan-treated knockouts, showing that CGRP release had behavioral consequences in MOH.
Laser Doppler blood perfusion scanning was then used to show increased meningeal blood flow in response to SNP injection into the jugular vein only in sumatriptan-treated wild-type mice and not in saline controls or sumatriptan-treated Nav1.9 knockouts.
Finally, degranulation of mast cells, a process by which these immune cells release substances like histamine and prostaglandin E2 (PGE2), which are capable of exciting nociceptors, was also implicated as a downstream consequence of Nav1.9 activation in MOH. Here, a mast cell stabilizer prevented allodynia in response to SNP in sumatriptan-treated wild-type mice, but had no effect in sumatriptan-treated Nav1.9 knockouts. A final set of experiments showed that histamine and PGE2 activated Nav1.9 in dural afferent neurons.
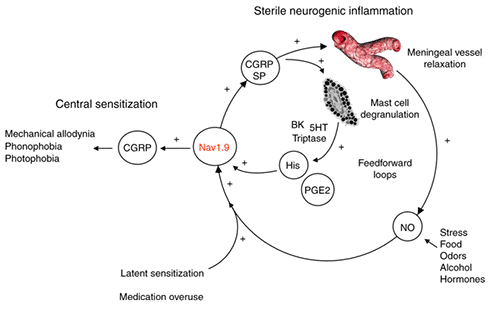
“The Nav1.9-induced release of CGRP and other vasoactive mediators from peripheral terminals initiates a cascade of events that include mast cell degranulation, further production of nitric oxide, and sustained sensitization of the trigeminal nerves,” Delmas summarized. “It really is a vicious cycle fueled by Nav1.9.”
The good news is that the identification of specific mechanisms and pathways involved in MOH points the way to new treatments for migraine sufferers.
“Subtype- and molecular-specific sodium channel blockers, in this case those that inhibit Nav1.9 channels, have the potential to be a more efficacious treatment for migraine, minimize side effects, and open up the possibility of combination and/or gene therapy,” said De Felice.
Lincoln Tracy, PhD, is a researcher and freelance writer based in Melbourne, Australia. Follow him on Twitter @lincolntracy.