Pain, including migraine pain, can be associated with sensory changes. It can also be an extremely unpleasant experience. But while the sensory characteristics of migraine pain have been examined closely in recent years, the unpleasantness of headache has been more difficult to study in animal models. New research seeks to address that gap in knowledge, identifying a brain circuit that mediates aversive behaviors in a headache model in rats.
Maggie Waung, University of California, San Francisco (UCSF), US, along with Howard Fields and colleagues, also at UCSF, show that headache activates a distinct neural circuit between the ventrolateral periaqueductal gray (vlPAG), an area implicated in pain modulation, and the ventral tegmental area (VTA), a vital part of the brain’s reward circuitry. What’s more, optogenetic deactivation of that circuit relieved the aversiveness of headache in the animal model.
“The value of this study is that it demonstrates it’s the aversive qualities of migraine pain that are being modulated by this circuit, not the sensory qualities of that pain,” said Frank Porreca, University of Arizona, Tucson, US. “The authors showed that there is an important aspect of migraine pain that, historically, has been overlooked but is deserving of our attention in the future as we try to understand more about what causes migraines,” according to Porreca, who studies migraine but was not involved with the new study.
The research was published September 10, 2019, in Cell Reports.
Direct connections between the vlPAG and VTA
The understanding of migraine has changed significantly in recent years, with the brain now taking center stage. Indeed, Waung said that symptoms such as sensitivity to sound, light, and smell point to a common neurological process in migraineurs.
“In medical school, I was taught that migraine was due to changes in cerebral blood vessels,” said Waung. “But once I became a neurologist, and spoke to more patients who had chronic headaches, it became clear to me that migraine is a disorder of the brain and how the brain processes stimuli. To better understand what might be triggering these headaches, we needed to take a brain circuit approach.”
To do that, the researchers first examined the neural connections between the vlPAG and VTA in rats. Using an adenovirus to deliver a fluorescent protein to label vlPAG projections to the VTA, they found that such nerve fibers were distributed throughout the VTA.
Next, the group used optogenetic methods to activate vlPAG inputs into the VTA, expressing the light-sensitive protein channelrhodopsin-2 (ChR2) in these projections. Whole-cell recordings revealed that, upon light activation, more than half of the VTA neurons received input from the vlPAG, with 68 percent of those responses having an excitatory component (measured as excitatory postsynaptic currents; EPSCs). About 50 percent of the neurons received inhibitory input, showing inhibitory postsynaptic currents (IPSCs) in response to light, and 26 percent of the VTA neurons with vlPAG connections received both excitatory and inhibitory synaptic input.
Together, the results showed that there are direct connections between the vlPAG and the VTA, with most VTA neurons receiving excitatory input from the vlPAG.
A role in headache?
To determine whether the vlPAG neurons projecting to the VTA might be important during headache, the researchers used a rat model in which they applied inflammatory mediators directly onto the dura, one of the membranes that surround the brain, to activate nociceptors there. This model mimics headache, including some of the features of migraine, such as allodynia and increased sensitivity to sound. Application of inflammatory mediators to the dura also increases the expression of Fos (an indirect measure of neuronal activation) in brain regions involved in headache.
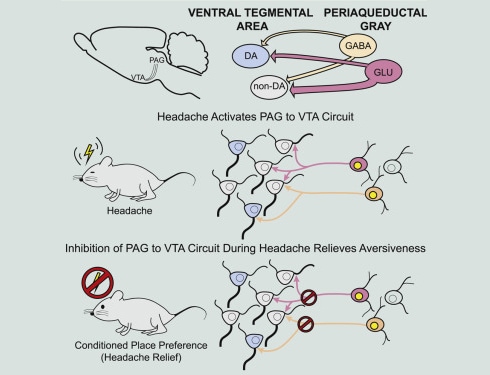
The team discovered that animals receiving dural inflammatory mediators showed a higher number of Fos-positive neurons in the vlPAG, compared to saline controls. This included a significant number of vlPAG cells that projected to the VTA.
The next step was to see whether activation of the vlPAG-VTA circuit affected behavior. Here, the researchers took advantage of optogenetic techniques to manipulate the activity of fibers originating from the vlPAG during a place preference assay. The group first found that otherwise healthy rats avoided the side of a chamber that was paired with blue light activation of vlPAG-VTA nerve terminals expressing ChR2.
The researchers then looked at how rats that had received dural inflammatory mediators to mimic headache would respond to manipulation of the vlPAG-VTA circuit. Here, the team used optogenetic inhibition to quiet vlPAG terminals in the VTA. In this case, animals that received dural inflammatory mediators developed a preference for the light-paired side of the chamber, compared to control animals that received dural saline and showed no preference. The results suggest, Waung said, that turning off the vlPAG-VTA circuit relieves the aversive state caused by headache.
“While the PAG has an important role in decreasing pain, there is growing evidence for its role in exacerbating pain, and, when you put it all together, it does make sense. This provides us a potential circuit to target for future migraine therapies.”
Waung added that, to date, the approaches researchers have used to study migraine in animals have been limited. But in the future, researchers can look to these circuits, as well as other key brain regions that are activated during headache, to not only better understand how different internal and external sensory stimuli may lead to migraines, but also to test potential drug therapies. Porreca agreed.
“We’ve always understood that migraine pain is mediated through central circuits in the brain,” he said, “but the study authors reveal that it is modulated through aversive input from the vlPAG—that’s quite new. And in doing so, they have identified potential targets within the brain that could potentially be accessible to new types of medicines to help reduce pain in migraine patients in the future.”
Kayt Sukel is a freelance writer based outside Houston, Texas.
A plain language summary of this news article is available on RELIEF, PRF's pain research news web site for the public. See here.