Fibromyalgia syndrome (FMS) ranks among the most enigmatic and prevalent chronic pain conditions. Researchers and clinicians have searched in vain for an underlying cause for the unexplained widespread muscle pain, fatigue, and tenderness. In recent years, FMS has come to be seen as a “central” pain disorder, arising from changes in pain processing in the central nervous system. Now several new reports show evidence for peripheral nerve abnormalities in FMS patients that could contribute to their chronic pain.
Jordi Serra of the University of Barcelona, Spain, and University College London, UK, said that together the studies—including his own work—suggest that at least some people with FMS have peripheral neuropathy and support the idea that FMS is a neuropathic pain condition. “Even though FMS is currently not considered a neuropathic disorder, it really looks the same” in some respects, Serra said.
Peripheral nerves damaged
One study, from Nurcan Üçeyler, Claudia Sommer, and colleagues at the University of Würzburg, Germany, compared 25 people diagnosed with FMS to 10 with depression but not pain, and similar numbers of matched healthy controls. The FMS group showed impaired peripheral sensory nerve function as measured by quantitative sensory testing—a subjective test—and by electrophysiological recording of pain-related evoked potentials. Skin biopsies revealed reduced innervation with the small fiber nerves that carry painful sensations in the legs. The group with FMS also reported higher levels of neuropathic pain symptoms. The study was published online March 9 in Brain.
In a separate study that appeared June 5 in Pain, Anne Louise Oaklander and her team at Massachusetts General Hospital, Boston, US, reported similar findings in a group of 27 people rigorously diagnosed with FMS: Skin biopsies from the legs revealed that 41 percent of the FMS patients had loss of small fiber innervation to levels considered clinically diagnostic for small fiber peripheral neuropathy (SFPN), a condition that can cause widespread pain. FMS patients also reported more symptoms than controls did on a questionnaire designed to detect neuropathic pain, and neurologic examination indicated more evidence of SFPN. The results led the investigators to suggest that some of the group diagnosed with FMS actually had unrecognized SFPN.
A third study presented at the November 2012 meeting of the International Association for the Study of Pain by Serra showed functional abnormalities in the small nerve fibers of FMS patients to match the anatomical differences seen by Oaklander and Sommer. Using a challenging technique called microneurography to record from individual nerve fibers in the skin, Serra had previously demonstrated hyperactivity in sensory neurons from patients with painful peripheral neuropathy (Serra et al., 2011). Serra found similarly altered conduction and firing properties in FMS patients compared to controls. That work is currently in press in the Annals of Neurology.
A condition by any other name?
The results raise the question of whether some patients diagnosed with FMS actually have SFPN. Sommer and colleagues stop short of calling their findings SFPN, instead describing “small fibre pathology” in the FMS patients. But Oaklander says making the diagnosis is important, because unlike FMS, which has no known cause and thus no disease-based treatment, small fiber polyneuropathies have some known causes that suggest treatment options.
In a letter to Pain commenting on Oaklander’s study, Üçeyler and Sommer argued that such a simple explanation is unlikely, because FMS includes some symptoms that are distinct from the usual presentation of SFPN (Üçeyler and Sommer, 2013). For example, many patients with FMS experience the burning pain or nerve tingling characteristic of SFPN, but most with SFPN do not experience the hallmark FMS symptom of widespread deep tissue pain. That discrepancy led Sommer and colleagues to conclude that the nerve damage seen in the epidermis probably doesn’t underlie the deep, widespread pain of FMS, which instead might result from similar problems in the small fibers that extend to muscles, fascia, and tendons.
In a published response, Oaklander pointed out that people with SFPN vary in their clinical presentation, which may include widespread pain (Oaklander et al., 2013). Her group previously reported finding juvenile-onset small fiber polyneuropathy in people with unexplained widespread pain, many of whom had previously received a diagnosis of FMS (see PRF related news story).
Oaklander and colleagues stress that making the diagnosis of SFPN in some FMS patients could lead physicians to look harder for an underlying cause—such as hepatitis, diabetes, or an immune response directed at sodium channels—and perhaps suggest alternative treatments. In the current study, the investigators looked for possible causes in 13 FMS patients with identified small fiber pathology and found two had hepatitis C, three had a potential genetic cause, and eight had markers of dysimmunity.
If an underlying cause for the peripheral nerve pathology could be identified, would it lead to improvement for FMS patients? “I like the optimistic stance,” said Roland Staud, a rheumatologist at the University of Florida, US. “I’d say yes; if you identify the main driving cause for their response, there’s a good chance they’ll [improve].”
Daniel Clauw, who studies fibromyalgia at the University of Michigan, US, disagrees. Clauw said that while the science in the new studies is sound, he doesn’t see how the findings should change treatment for people with FMS. Until that happens, he said, “I hang my hat on CNS treatments” that modulate pain-processing circuits. Clauw went a step further and suggested that the observed nerve damage probably follows FMS rather than the other way around. “It may be a marker of a chronically overactive nervous system,” Clauw said. The central nervous system undergoes remodeling with chronic pain, he said, so why not the peripheral system as well?
One hypothesis to explain FMS is that people with the disorder become, for unidentified reasons, extremely sensitive to nociceptive input, possibly because peripheral sensory signals not normally felt as painful become amplified in the brain and spinal cord. Whereas many people with identified peripheral nerve damage do not experience any neuropathic pain, people with FMS could have an enhanced response to such damage.
How that amplification is set into motion remains a mystery, but FMS often develops following a traumatic event or injury, and patients might have several other concomitant pain conditions. “Fibromyalgia can be triggered or exacerbated by multiple conditions, and now it appears that small fiber neuropathy may be one of them,” said Staud.
Another peripheral trigger?
In other related work published May 20 in Pain Medicine, Frank Rice and colleagues at the Albany Medical College, New York, US, found different peripheral nerve abnormalities in the palms of FMS patients. Tiny blood vessels called arteriole-venule shunts (AVSs) in the hands are important for regulating body temperature and controlling blood flow to muscles during exercise. The shunts constrict in response to norepinephrine released by autonomic fibers and dilate in response to peptides released by sensory fibers, giving these afferents a dual role as receptors and effectors. Rice found that the fibers were significantly altered in the FMS patients but not in control subjects. Innervation by both fiber types was increased with FMS, and the balance was tipped so that sensory neuron endings outnumbered autonomic fibers.
Rice postulates that the imbalance might affect thermal regulation or produce deep tissue ischemia, perhaps contributing to widespread muscle pain or fatigue. Staud appreciates the findings but said, at this stage, “the relationship to fibromyalgia is not at all clear.” Next, said Staud, experiments will be needed to correlate the anatomical findings with pain or other aspects of FMS. “I’m totally on board if he shows evidence of that,” said Staud, but “otherwise, it is an oddity that we cannot currently explain.”
Despite their different interpretations of the recent findings, the researchers all agreed that changes in the central nervous system certainly contribute to FMS to some degree. “We are not saying the CNS is not important,” said Serra. “We are only saying that, because [pain] seems to be coming from the periphery, it makes sense to look there.”
Staud agreed and said that in treating FMS patients, “I look for potential biological causes that could drive it from the periphery,” and these findings present another potential cause. What the findings will mean for FMS treatment remains to be seen.
Stephani Sutherland, PhD, is a neuroscientist, yogi, and freelance writer in Southern California.
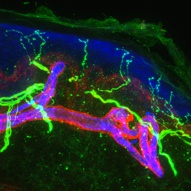
Image: A 60-micron optical section of human skin, showing epidermal nerve fibers (thin green) penetrating into the epidermis (blue). Credit: William Kennedy and Gwen Wendelschafer-Crabb, University of Minnesota, Minneapolis, US.