Editor’s note: Stephen McMahon, PhD FMedSci (1954-2021), was a world-class researcher, lecturer, and inspiration to many. “Mac” worked relentlessly, and his last electrophysiology paper, presented within this news article, was published in August 2021. He will be remembered not only for his seminal contributions to our understanding of pain, but also for his generous and enthusiastic personal spirit, which has left an indelible mark. We encourage all of our PRF readers to appreciate the memorials written for Stephen from Nature Neuroscience, IASP, PAIN, and King’s College London, UK. Additionally, consider listening to The Pain Beat (Episode 12) – A Tribute to Stephen McMahon, where podcast guests pay homage to “Mac” with stories that illustrate his influence on the pain research field and those in it.
Blocking nerve conduction in injured nerves is one of the most effective ways to treat clinical pain. The concept is simple: Block the signals that cause chronic pain at their source, be it in the skin or the nerve root, but the method is obviously more complex. A major challenge thus far has been finding ways to selectively block the signals that result in chronic pain without altering somatosensation, a side effect of administering local anesthetics, such as lidocaine.
A recent publication has demonstrated a new method to inhibit peripheral neuronal activity and reduce chronic pain in patients via neuromodulation. The research, led by Stephen McMahon, King’s College London (KCL), UK, shows that stimulating the dorsal root with ultra low frequency (ULF) currents causes a reliable and safe blockade of nerve conduction in a large population of peripheral nerves. Furthermore, patients with chronic low back pain reported a dramatic reduction in pain levels in a small-scale clinical study.
“ULF is a unique way of blocking action potentials in sensory neurons, which shows promising but preliminary efficacy in reducing pain in patients. If future clinical studies examining ULF show clinical efficacy, then adding this waveform to the toolbox could help a lot of patients,” remarked Benedict Alter, University of Pittsburgh, US, who was not part of the study.
The study was published in Science Translational Medicine on 25 August 2021.
ULF waveform: a new way to inhibit nerve conduction
Neuromodulatory technology is not a new method to treat pain. It was first developed in the 1960s following Melzack and Wall’s gate control theory of pain (Melzack and Wall, 1965). The idea was that clinicians could relieve pain by stimulating large-diameter primary afferent neurons, thereby activating inhibitory networks in the spinal cord, which dampen nociceptive transmission and pain.
“In the clinic, neuromodulatory devices like spinal cord stimulation (SCS) devices are often used as last options for pain treatment if patients are unresponsive to conventional treatment options. But they are an indirect way to treat pain, as you’re relying on inhibitory networks in the central nervous system,” explained author Scott Lempka, University of Michigan, US.
The story began when Stephen McMahon and colleagues at KCL established a collaboration with Presidio Medical, a medical technology company based in the US. Presidio Medical has been working on novel neuromodulatory technologies that inhibit nerve transmission, instead of activating nerve fibers.
“The technology from Presidio Medical has really moved neuromodulation technology on,” expressed first author Martyn Jones, Zenith NeuroTech Ltd. and KCL, UK. “People have known for years that you can block nerve conduction if you apply current to nerve tissue, but doing this safely for the long periods necessary to make it clinically useful has not previously been possible. Presidio Medical’s novel technology is different because the slowly alternating ULF waveform reduces action potential firing safely and reversibly."
The device uses the ULF current to inhibit nerve transmission. Essentially, ULF is like direct current, but it alternates its polarity at regular intervals with slowly cycling waveforms (with a period of ~0.1 Hz), so the current is not maintained in one direction. The fact that the waveform is cycling, coupled to its low current amplitude requirement, means that conduction in a large number of nerve fibers can be attenuated over sustained periods of time without causing tissue damage.
Blocking ectopic firing at the source
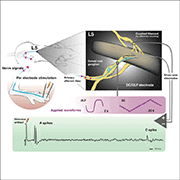
As proof of principle, the authors first explored how effective the ULF waveform was at inhibiting action potential firing in peripheral nerves in an animal model. They began by recording activity in individual axons in the L4 or L5 dorsal roots in anesthetized rats in response to electrical stimulation of the neuronal receptive fields in the paw. At the same time, ULF currents were applied to the nerve root via a separate electrode.
The ULF waveform had an impressive effect, blocking action potential conduction in 98% of non-nociceptive Aß-fibers, 82% of Aδ-fibers, and 46% of C-fibers. For many afferents, blocking was immediate and typically seen in the anodal, not cathodal, phase, and these fibers resumed firing four or five minutes after ULF had been turned off. However, a different population of fibers had a more gradual reduction of firing before total block, with a delayed recovery after ULF was turned off.
“In uninjured animals, ULF was very effective in inhibiting nerve transmission. Interestingly, it seems to be more efficacious in larger-diameter fibers that are not traditionally associated with transmission of nociception, but of course that may change in pathological circumstances. It may be that those fibers with low ULF thresholds are not much involved in normal nociception but could contribute to chronic pain under neuropathic conditions,” Jones told PRF.
With this hypothesis in mind, the authors studied the effects of ULF waveform conduction block in neuropathic pain, modeled in rats by axotomizing the L5 spinal nerve. A hallmark of nerve injury is spontaneous ectopic firing in the injured nerve fibers, a defect that is thought to drive paresthesia and spontaneous pain in neuropathic patients.
In their model, injury-induced ectopic activity was recorded up to eight days after injury. However, when ULF current was applied to the dorsal root, the ectopic firing was reduced. Ectopic firing activity took 20 minutes of sustained ULF current to be blocked, but had a time lag of 17 minutes to recover after ULF was switched off.
“We thought that this delay in the return of ectopic activity after ULF stimulation could be interesting in clinical terms, so a patient doesn’t have an immediate return of pain if the device is switched off,” explained Jones.
Stopping action potentials in their tracks
Next, the authors developed a computational model to investigate how ULF currents change the biophysical properties of axons.
“The idea was that we could create a well-controlled environment to tease apart how ULF is affecting the nervous system, as well as the design of the electrode system. For this, we used a multicompartmental axon model with a cylindrical extracellular region. We could use this model to mimic the experimental conditions we saw when ULF effectively blocked nerve transmission in animal models,” Lempka told PRF.
This computational model showed the same two types of ULF-driven conduction block seen in the animal model: the immediate and gradual blocks. It also revealed that the immediate conduction block was due to near-complete inactivation of voltage-gated sodium ion channels during the plateau phases of the waveform. This coincided with inactivation of sodium ion channels. The delayed nerve block, on the other hand, was due to the more gradual accumulation of potassium ions in the extracellular space, leading to a depolarizing shift in the axon, sodium ion channel inactivation, and eventual action potential conduction block.
While this computational model revealed a mechanism of conduction block at the single-cell level, Jones emphasized that more needs to be understood on a systems level. “One area we continue to explore is isolating the exact neuronal pathway ULF is blocking to inhibit pain. We know from our animal studies that ULF blocks primary afferent neuron conduction, but we’re also doing work to see if ULF directly and/or indirectly inhibits activity in the spinal cord or other nerve tracts,” Jones said.
ULF takes the oof out of back pain
Naturally, these animal and computational results led the authors to question whether ULF current stimulation would be an effective treatment for chronic pain in patients. Here, the authors brought in Marc Russo from the Hunter Pain Clinic, NSW, Australia, to conduct a small clinical study of 20 subjects presenting with chronic low back pain.
After the ULF device was implanted, subjects reported their pain levels on the visual analogue scale (VAS) over the 15-day study period. Once switched on, the ULF waveform had a remarkable effect on reducing pain levels. Pre-screening pain levels were an average of 75 on the VAS, but fell to 15.7 by day seven and 7.6 by day 15 following ULF exposure (a 90% improvement in pain levels over 15 days). Moreover, seven patients achieved 100% back pain relief, and 18 achieved 80% pain relief, demonstrating the efficacy of ULF stimulation.
“I was surprised to see that in the open-label clinical trial there was a significant pain reduction without any reported alteration in sensation. If pain relief from ULF treatment requires conduction blockade, then we would expect altered sensation in patients, but this didn’t occur. However, it could be possible that some of the pain relief observed is due to the placebo effect, given that there was no control group to test this,” commented Alter.
The effects of ULF were not permanent, however. After the stimulatory devices were removed on day 15, subjects reported that the pain returned within 24 hours. Nevertheless, this early clinical study showed just how efficacious ULF was at treating chronic low back pain in patients.
“In our preclinical studies, ULF inhibited nerve signals in models of pain in the laboratory in a way we haven’t really seen with other technologies. The fact that ULF then had such a strong effect on reducing pain levels in patients was very exciting. We know that the implant and current stimulation protocols are safe, meaning it could be used for treating other types of chronic pain, and even other diseases. We still have a lot to do, but it certainly looks promising for the future,” expressed Jones.
Fred Schwaller, PhD, is a freelance science writer based in Germany.
Feature image: Experimental setup for stimulation, recording, and application of ULF current. Jones et al., Science Translational Medicine. 2021 Aug; 13.