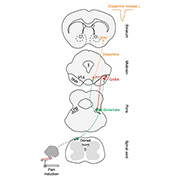
In addition to reward and motivational processing, dopamine (DA) dysfunction is also associated with many chronic pain conditions. It is known that pain reduces the activity of DA neurons in the ventral tegmental area (VTA) (Ungless et al., 2004), and a recent study led by Stephan Lammel, University of California, Berkeley, US, illustrates the neural circuit and mechanism responsible for the decrease in DA release following noxious insults.
When describing the rationale behind the study, first author Hongbin Yang, Zhejiang University, China, told PRF, "The activity of many DA neurons in the VTA is decreased in response to noxious stimulation, but they do not receive direct inputs from the spinal cord, so there must be a mediator between the spinal cord and the VTA that is responsible for these changes in DA signaling.” Previous studies discovered functional connectivity between the spinal cord and the lateral parabrachial (LPB) nucleus (Cechetto et al., 1985), and that LPB neurons respond to a wide range of noxious stimuli (Bernard et al., 1994). Therefore, the team focused on deciphering the role of LPB projection neurons in modulating pain-related DA response.
"This is a really elegant paper describing the neural circuitry of DA signaling, which is normally associated with reward and error prediction. Painful stimulation in this context may represent expectations of a negative outcome; thus, understanding the mechanisms may shed light on what the brain is doing when an individual is learning to avoid pain,” Stephanie Borgland, University of Calgary, Canada, commented on this work.
This study was published on August 9, 2021, in Nature Neuroscience.
Midbrain dopaminergic neurons receive input from the LPB nucleus
Using viral tracing to map neuronal projections in mice, the team first showed that the LPB nucleus in the midbrain forms non-overlapping connections to two mesencephalic regions of the brain: the substantia nigra pars reticulata (SNR) and the VTA. Next, the team showed that neurons in the spinal cord dorsal horn project directly to the SNR and LPB, but preferentially target SNR projecting LPB neurons.
Using a combined optogenetic and patch-clamp electrophysiology approach, the team next explored the functional connectivity among LPB, SNR, and VTA cells. Under light-stimulated channelrhodopsin-2 (ChR2) activation, the authors detected large excitatory postsynaptic currents (EPSCs) in all SNR and VTA neurons. Additionally, using viral tracing to label vesicular glutamate transporter 2 (vGLUT2) and synaptophysin, the authors found LPB terminals in the SNR and VTA to be excitatory. These data posed an interesting conundrum, as Yang told PRF, “this suggests to us that most cells in the LPB are glutamatergic. So how does an excitatory cell play a role in blocking dopamine release, presumably via inhibitory mechanisms?”
To solve this puzzle, the team discovered that SNR neurons receiving inputs from the LPB express vesicular GABA transporter (VGAT), suggesting that the direct output of LPB to SNR neurons is inhibitory. In parallel, the team observed that a subpopulation of VTA cells, the GABAergic neurons, also receive LPB inputs. However, functional connectivity could not be established using patch-clamp electrophysiology, which led the team to hypothesize that the LPB also decreases DA transmission via an indirect mechanism. By simultaneously recording electrical signals in VTA terminals receiving LPB inputs, and optogenetically stimulating SNR terminals receiving LPB inputs, large inhibitory postsynaptic currents (IPSCs) were detected at VTA terminals. Altogether, a new parabrachial-mesencephalic circuit is proposed whereby GABA-expressing SNR neurons modulate DA activity either through direct inhibition onto DA-expressing neurons in the VTA, or indirectly by looping back to the LPB and reducing the excitability of VTA-projecting LPB neurons.
Ascending pain signals travel through the parabrachial-mesencephalic circuit
After the identification of this parabrachial-mesencephalic circuit, the team turned their attention to quantifying the behavioral significance of this pathway in a variety of pain models. The team focused on SNR projecting LPB neurons, as they are preferentially targeted by ascending spinal projections. Using fiber photometry, the researchers observed increased activity in SNR projecting LPB neurons during the delivery of electrical shock, tail pinching, and heat, confirming that SNR projecting LPB neurons are excited by painful stimuli. As fiber photometry only allows for population-based cellular activity measurements, the team added single-cell level analysis by incorporating optogenetic-cellular tagging methods (using Cre-driven expression of ChR2 in SNR projecting LPB cells and activating ChR2 with a drivable optoelectrode). Taken together, these complementary techniques showed that noxious stimuli excite SNR projecting LPB neurons.
Following the injection of an adeno-associated virus carrying halorhodopsin into SNR projecting LPB neurons, the team was able to silence these projections and measure pain behaviors in both inflammatory and neuropathic pain models. In the formalin model of pain, which exhibits two distinctive phases (i.e., phase I pertains to the first 10 minutes of licking, paw lifting, and flicking that transpire before these behaviors subside, followed by phase II in which these behaviors re-emerge and can last up to an hour), inhibition of SNR projecting LPB neurons blocked phase II behavioral responses. Similarly, mechanical and thermal hypersensitivity was reduced after the silencing of SNR projecting LPB neurons in the sciatic nerve ligation (SNL) model of neuropathic pain. To avoid confounding results on animal behavior following these optogenetic cellular manipulations, the team ran a complementary loss-of-function experiment by virally inducing apoptosis with Cre-dependent caspase 3 in SNR projecting LPB neurons. Similar observations were made whereby the programmed cellular death of SNR projecting LPB neurons alleviated phase II formalin pain behaviors.
DA signaling completes the ascending parabrachial-mesencephalic circuit
Finally, the team was able to show that SNR projecting LPB neurons are necessary for the inhibition of DA release upon pain stimulation. In a transgenic mouse line that tags DA neurons (DAT-Cre), fiber photometry data showed that DA activity was reduced following the stimulation of LPB terminals in the SNR. Similarly, in a naive mouse strain (C57/BL6), the ablation of LPB projections using virally delivered caspase 3 reversed the impact of tail electrical shock and pinch on DA release, whereas the researchers observed the inhibition of DA release in animals receiving a control virus.
Taken together, these data showed that blocking the activity of SNR projecting LPB neurons leads to a reversal in pain behaviors and rescues DA function. The findings improve our understanding of how the brain receives painful information from the periphery, and proposes the importance of the LPB neural circuit in pain signaling via DA.
Understanding the physiological context of neural circuitry
Describing the novelty of this study to PRF, Yang commented, “The SNR, as part of the basal ganglia circuitry, is well known to control motor behaviors, yet its role for sensory and pain modulation is less understood.” This study alludes to the LPB as a key brain region for sensory and motor interactions. The anatomical and functional dissection of this spinal-parabrachial-mesencephalic pathway could also pave the way for others to understand how neural circuits are assembled to generate behaviors. The LPB projects to many different brain nuclei (aside from the SNR and the VTA, the LPB also projects to brain centers such as the amygdala and the periaqueductal grey), and it is possible that there are other, parallel pathways that modulate pain or other comorbid pain behaviors including fear or aversive learning.
Borgland added, “In this study the authors found that LPB-targeted SNR neurons also project to the dorsal striatum, an area known to be important for motor control. In the future, it would be interesting to further understand the input/output relationships between these dopaminergic regions and test whether the circuity identified can be generalized to other types of aversive behaviors, as well as pain.”
Charlie Kwok, PhD, is a postdoctoral fellow at Hotchkiss Brain Institute, University of Calgary, Canada.
Image: Schematic illustrating a neural circuit conveying nociceptive input from the dorsal horn spinal cord to midbrain DA neurons projecting to the nucleus accumbens lateral shell. Credit: Yang et al. Nature Neuroscience. 2021; 24:1402-1413.