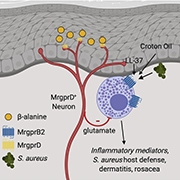
Research published in Cell on April 15, 2021, found that a specific subset of neurons, MrgprD-expressing neurons, suppress mast cell hyperresponsiveness in the skin and work to maintain skin homeostasis. Furthermore, the survival of these MrgprD-expressing neurons is dependent on the presence of Langerhans cells. This cellular communication pathway is critical for understanding how the nervous system can control mast cell-driven inflammation (see related PRF Paper of the Week), and this research was performed by the lab of Daniel Kaplan at the University of Pittsburgh, US.
Xinzhong Dong at Johns Hopkins University School of Medicine, Baltimore, US, thought the work produced “really interesting findings,” and that this work “provides a new mechanism for nonpeptidergic fibers and [how] they can actually regulate the immune response.”
There are three leading players in this study: Langerhans cells, mast cells, and MrgprD-expressing neurons. Langerhans cells are epidermal-resident antigen-presenting immune cells. Functionally, there is considerable overlap between Langerhans cells and dendritic cells, but they are most closely related to macrophages.
Connective tissue mast cells are skin-resident innate immune cells that play an essential role in allergic reactions and inflammation. Most people know them as the immune cells that produce histamine, which is released along with other inflammatory mediators, during a process called degranulation. Mast cells express MrgprB2 (Mas-related G protein-coupled receptor B2), a receptor required for mast cell activation and degranulation.
MrgprD-expressing neurons (Mas-related G protein-coupled receptor D) innervate the skin and are thought to be involved in the sensation of itch, pain, and mechanical stimuli. They also play a role in inflammatory pain. However, prior to this research, their role in immune modulation had not been deeply investigated.
This research group used various transgenic mouse lines that allowed for targeted depletion of neurons and immune cells. The three prominent mouse lines used extensively throughout these studies were Human Fiber-DTA (called LCDTA), Human Langerin-DTR (called LCDTR) and MrgprdDTR. The use of LCDTR and MrgprdDTR mice allows for the inducible depletion of Langerhans cells or MrgprD-expressing neurons, respectively, through the administration of diphtheria toxin (DT). In contrast, Langerhans cells are ablated in LCDTA mice without the need for the administration of another compound.
There were two main skin challenges used in this experimentation: croton oil and S. aureus. Croton oil applied to the ears of mice causes swelling/thickening and is used as a model of contact dermatitis. S. aureus is a Gram-positive bacterium that produces factors which can act directly to activate unmyelinated C-fibers and result in the sensation of pain. When S. aureus is injected intradermally to the flank, it causes the formation of a skin lesion.
Evans blue extravasation was also used throughout this study and tests for blood vessel permeability. Mice are anesthetized and intravenously injected with Evans blue dye. Healthy, naïve mice will have minimal to no blue discoloration in surrounding tissues, whereas mice with permeable blood vessels, a result of swelling or inflammation in the surrounding tissues, will present with blue discoloration.
Langerhans cells influence mast cells through MrgprD-expressing neurons
Following the application of croton oil to the ears of wild-type mice, swelling would occur. However, if Langerhans cells were knocked out (using the LCDTA mouse model), there was significantly more swelling. It was also discovered that when Langerhans cells were depleted for three days (using the LCDTR mouse model), there was no observable increase in ear swelling in comparison to wild-type mice; however, there was a significant increase in the ear swelling of mice in which Langerhans cells were depleted for 30 days. This ablation of Langerhans cells was coupled to the observation of increased mast cell degranulation.
During long-term Langerhans cell depletion, there was increased leaking of Evans blue dye into surrounding tissues (evidence of increased blood vessel permeability). When either mast cells or Langerhans cells and mast cells were depleted, a similar reduction of ear swelling was observed in comparison to when only Langerhans cells were depleted. Substance P (TAC1) is known to activate MrgprB2, a receptor required for mast cell degranulation. When croton oil is applied to the ears of Tac1 or Mrgprb2 knockout mice, the intensity of ear swelling is decreased. In mast cells isolated from the ear of LCDTA mice, there was a significant increase in Mrgprb2 mRNA expression compared to wild-type or LCDTR mice. There was also heightened leaking of Evans blue dye in LCDTA mice when Mrgprb2 was activated. These results suggested a link between the presence of Langerhans cells and the activation of mast cells.
Mice that experienced long-term depletion of Langerhans cells showed a reduction in nerve fiber density and fewer nonpeptidergic neurons. Using immunohistochemistry, mast cells were observed clustering near MrgprD-expressing neurons. When mice that lacked MrgprD-expressing neurons were studied (the MrgprdDTR mouse model), they had increased ear swelling after croton oil application compared to wild-type mice. When dermal mast cells were isolated from these MrgprdDTR mice, an increase in Mrgprb2 was observed. When mast cell degranulation was stimulated, mice lacking Mrgprd showed heightened Evans blue dye leaking into surrounding tissue. Both Shiqun Zhang and Kaplan noted their surprise after discovering the role MrgprD-expressing neurons play in mast cell suppression.
Mice were then challenged with S. aureus to induce skin lesions. Mice lacking MrgprD-expressing neurons or Langerhans cells showed decreased lesion size, whereas mice lacking mast cells showed an increased lesion size.
Increasing the excitability of MrgprD-expressing neurons
β-alanine was used to activate and enhance the excitability of MrgprD-expressing neurons. Less Evans blue dye leaked into the surrounding tissue of mice when MrgprD-expressing neurons were activated, even when degranulation of mast cells was concurrently stimulated. β-alanine also resulted in a decrease in Mrgprb2 mRNA expression in the flank skin. Using the S. aureus model, the researchers observed that mice administered β-alanine showed an increase in lesion size. Following croton oil application, mice administered β-alanine showed a decrease in ear swelling. These results suggested that when MrgprD-expressing neurons are activated, mast cells are suppressed.
RNA-seq was used to better understand the mechanisms behind the interaction of Langerhans cells, mast cells, and MrgprD-expressing neurons. Ear skin from LCDTR, LCDTA, MrgprdCre, and MrgprdDTR mice, as well as mice treated with PBS or β-alanine, were used to determine the gene modules that could be responsible for the differences in ear swelling following the application of croton oil. A gene module was identified with similar results in comparison to these phenotypic observations and involved in mast cell activation. To determine if the genes identified from whole ear skin could also be reflected in isolated mast cells, RNA-seq was performed on isolated mast cells from MrgprdCre mice and DT-treated MrgprdDTR mice. The genes that were both differentially expressed in the purified mast cells and the selected gene module were displayed in a heat map. These results suggested that mast cells were downstream in the communication between Langerhans cells and MrgprD-expressing neurons.
The role of glutamate
qPCR of whole dorsal root ganglia (DRG) was carried out on wild-type, LCDTA and MrgprdDTR mice to understand how MrgprD-expressing neurons alter mast cells. Slc17a6, the gene for the vesicular glutamate transporter vGlut2, was significantly reduced in the DRG of LCDTA mice. There was also an increase in Slc17a6 in the DRG for mice administered β-alanine. To determine if glutamate release from MrgprD-expressing neurons regulates mast cell function, a mouse line was engineered called MrgprdΔvGlut2/+ that carries a loss of a single Slc17a6 allele in MrgprD-expressing neurons. These mice have reduced glutamate release without generating potential developmental defects. Mrgprb2 was increased fourfold in naïve MrgprdΔvGlut2/+ skin. When mast cells were stimulated to degranulate, Evans blue dye extravasation in MrgprdΔvGlut2/+ mice was heightened compared to wild-type mice. This suggested that glutamate released from MrgprD-expressing neurons reduces the activation of mast cells during homeostasis.
Glutamate receptors were selected out from the RNA-seq dataset of dermal mast cells from MrgprdDTR and control mice. Grik2 (GluR6) and Grik5 (KA2) showed the highest expression in this dataset. Interestingly, KA2 is known to form a heterodimer with GluR5-7. Therefore, the authors theorized that the most likely glutamate receptor on mast cells is a heterodimer of KA2 and GluR6. They next used a GluR6 selective antagonist named NS102 to understand the role this heterodimeric receptor plays in mast cell function. Mice that were administered NS102 displayed an increase in Mrgprb2 mRNA in the flank skin. Furthermore, mice that were given NS102 and β-alanine showed the same relative mRNA expression as those who only received NS102, suggesting that NS102 is acting downstream of the glutamate response.
To investigate if glutamate can act directly on mast cells, PCMCs (peritoneal cell-derived mast cells) were purified from wild-type mice and cultured for experimentation. β-hexosaminidase was used to indicate the amount of degranulation. PCMCs cultured with NS102 showed a dose-dependent increase of Mrgprb2, and increased β-hexosaminidase release after degranulation was stimulated. RNA-seq was performed on mRNA isolated from PCMCs cultured with NS102 or a vehicle control to complete the study. When the differentially expressed genes in this dataset were compared to the upregulated genes from the sorted dermal mast cells from MrgprdDTR mice, a robust positive enrichment was observed. The authors theorize that inhibition of glutamate signaling, through the KA2/GluR6 heterodimeric receptor complex, programs a hyperactive mast cell state that strongly resembles the hyperactive mast cell state due to the loss of MrgprD-expressing neurons.
Next steps
The investigators have demonstrated the link among Langerhans cells, MrgprD-expressing neurons, and mast cells. However, it is still unclear why the long-term loss of Langerhans cells results in the depletion of MrgprD-expressing neurons. The authors hypothesize that these neurons require a Langerhans cell-derived growth factor, and that long-term absence of this factor could result in the retraction of the nerve endings. However, more work is needed to determine what this factor could be. An alternative Langerhans cell-depletion method, the LangerinDTR mouse model, shows a reduction of CGRP-expressing neurons and reduced expression of glial cell line-derived neurotrophic factor (GDNF) (Doss and Smith, 2021). GDNF is a GFRα2 ligand, and MrgprD-expressing neurons are dependent on GFRα2 ligands to maintain nerve endings.
Dong also suggests that “whether glutamate can inhibit mast cell expression of MrgprB2 still needs to be more firmly established.”
Additional work is also needed to determine how these neuro-immune interactions could be altered during infection, inflammation, and wound healing. Although the communication between MrgprD-expressing neurons and mast cells is clearly established, more investigation is needed to determine “whether this nonpeptidergic-mast cell regulation really contributes to pain,” notes Dong.
The researchers are eager to understand these interactions with a high degree of detail. Kaplan explains that “one of the important questions which we have not yet addressed is whether or not MrgprD-expressing neurons modulate mast cell reactivity dynamically. In other words, so far, we have defined a tonic inhibition under homeostatic conditions. I think it’s likely that once the state of the skin is perturbed by infection or inflammation that MrgprD-expressing neurons may play a role in tuning the response via mast cell reactivity. This might provide an explanation for the evolutionary advantage of this complex intracellular neuro-immune circuit.”
Zhang is hopeful that “understand[ing] the mechanism or function of MrgprD during inflammation or in [a] pain model will be helpful for [providing patients pain relief].”
Courtney Bannerman is a PhD Candidate at Queen’s University in Kingston, Ontario, Canada.
Graphical abstract. Zhang at al., Cell. 2021 April 15; 184, 2151-2166.