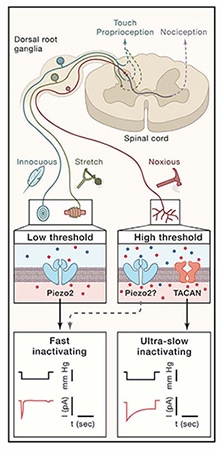
Mechanotransduction is the process by which a mechanical stimulus is converted into an electrical signal, allowing us to hear, feel touch, and experience pain, among other sensations. But much remains to be learned about mechanical pain, the most unpleasant type of mechanosensation. New evidence now puts forth a novel ion channel as a key sensor of mechanical pain.
Researchers led by Reza Sharif-Naeini, McGill University, Canada, call their newly identified ion channel TACAN – which means “movement” in Farsi – and demonstrate that it responds to membrane stretch in heterologous systems as well as in nociceptors. Further, the channel contributes to mechanical pain sensation but not to thermal or touch sensation, in vivo, in mice.
“This work is a breakthrough for the field, as the authors discovered a new ion channel involved in punctate pressure-evoked pain,” said Qiufu Ma, Harvard University, Boston, US. “However, the field still has a long way to go to fully understand mechanical pain, as this is just the tip of the iceberg,” according to Ma, who was not involved with the research.
“Overall, the exciting findings of [the authors] expand our understanding of molecular pain mechanisms and open intriguing avenues for future investigations,” according to an accompanying Preview by Oscar Arenas and Ellen Lumpkin, University of California Berkeley, US.
The research and Preview were published March 5, 2020, in Cell.
Zeroing in on TACAN
“As part of my postdoctoral work we performed a proteomic screen of membrane proteins involved in mechanotransduction using smooth muscle cells,” said Sharif-Naeini, explaining his path to the new study. “This screen led to a potential list of 70 candidates. We narrowed down the candidates to five by looking specifically at only transmembrane proteins of unknown function. One of these candidates we named TACAN.”
Tissues routinely exposed to mechanical stimuli, including the lung, heart, intestines, and dorsal root ganglia (DRG) sensory neurons, showed higher expression of TACAN in the new study. Focusing on the last, the researchers, including co-first authors Lou Beaulieu-Laroche, an undergraduate at the time, and postdoctoral fellow Marine Christin saw high expression in small- to medium-diameter DRG neurons.
The group also found that TACAN was predominantly expressed in mouse non-peptidergic nociceptors. Further, 80% of neurons expressing tyrosine hydroxylase, a marker of low threshold C-fibers, also expressed TACAN.
“It is noteworthy that TACAN was not expressed in peptidergic nociceptors, which include A-delta nociceptors for pinprick-evoked sharp pain and silent nociceptors for pinch-evoked sustained mechanical pain in response to tissue injury,” said Ma. “Additionally, this data leads me to wonder if TACAN might not be expressed in mechanical nociceptors that innervate deep tissues, such as joints, muscles, bones, and visceral organs,” he added, since TACAN-expressing neurons that also express Mrgprd- and tyrosine hydroxylase nearly exclusively innervate the epidermis of the skin or hair follicles (Mrgprd is a marker of non-peptidergic neurons; see below).
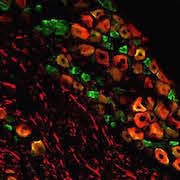
![TACAN Is Expressed in Nociceptors. Double immunostaining to determine colocalization of TACAN with markers of myelinated afferents (Neurofilament 200 [NF200]), peptidergic (CGRP) and nonpeptidergic (P2X3) nociceptors, C-type low-threshold mechanoreceptors (C-LTMRs; tyrosine hydroxylase [TH]), and GINIP (C-LTMRs and non-peptidergic nociceptors). Colocalization with proprioceptors was done by staining DRG sections of Pvalb:cre; tdTom mice with the TACAN antibody. [PV: parvalbumin]. Figure and caption from Beaulieu-Laroche et al. Cell. 2020 Mar 5;180(5):956-967.e17, with permission from Elsevier.](https://www.iasp-pain.org/wp-content/uploads/2023/02/TACANInline-image.jpg)
TACAN enhances cellular mechanosensitivity
To learn if TACAN was in fact mechanically sensitive, the investigators first tested its function in heterologous systems. Here, they patch clamped Chinese hamster ovary (CHO) cells expressing TACAN, using a cell-attached configuration (where the cell stays attached to the patch pipette, forming a tight seal without rupturing the membrane). This approach allows for exposure of the membrane under the pipette to pulses of negative pressure, which activates mechanically gated ion channels.
CHO cells expressing TACAN exhibited significantly larger mechanically evoked currents, compared to mock-transfected CHO cells. Although the activation threshold of the cells did not change, the percentage of active patches was significantly higher in TACAN-transfected CHO cells, which the researchers say reflects a higher density of mechanosensitive ion channels in those cells. The authors confirmed the increase in mechanically evoked currents in human embryonic kidney (HEK) 293 cells transfected with TACAN as well as in a fibroblast-like cell line.
Interestingly, the group did not observe TACAN-dependent mechanically evoked currents when cells were patch clamped in a whole cell configuration and poked with a second pipette. “This is most likely due to TACAN having a high activation threshold, which is very hard to reach in flat cell lines in a culture dish,” said Beaulieu-Laroche. “The high indentations most likely perforate the membrane before the activation threshold can be reached.”
To further investigate TACAN’s mechanical sensitivity, the researchers collaborated with study co-author Kate Poole, University of New South Wales, Australia, using a pillar array assay. Here, cells are grown on top of microscopic elastic cylinders (pili) that can be manipulated to apply very fine physical stimuli to the cells plated atop.
Serial deflections of elastic pili in close proximity to a cell produced mechanically evoked currents in eight out of eight HEK293-Piezo1 knockout cells expressing TACAN, while nine out of nine mock-transfected cells showed no such currents (Piezo1 is a mechanically sensitive ion channel, and the researchers used the HEK293-Piezo1 knockout cells to eliminate the contribution of that channel in the experiments). Intriguingly, the kinetics of these currents matched those previously measured in nociceptors, where pillar deflection elicits rapidly, intermediately, and slowly adapting current profiles in a single cell and requires high-threshold deflections (Poole et al., 2014).
Is it an ion channel?
Because TACAN expression increased mechanosensitivity in the cell lines, the authors next wanted to test whether TACAN was an actual ion channel. To do so, they expressed TACAN in bilayers formed from synthetic lipids and measured the current flow of ions across the membrane in response to different voltages. The addition of TACAN-containing proteoliposomes produced large currents, demonstrating that TACAN itself can form a pore across which ions can flow.
“We were very excited about these results and immediately wanted to test whether known blockers of mechanically gated ion channels such as gadolinium or the peptide GsMTx4 [a mechanosensitive ion channel blocker purified from the venom of a Chilean rose tarantula, Grammostola spatulata] could block these currents when we utilized concentrations known to work on endogenous mechanical currents,” said Sharif-Naeini. “Therefore, I took these blockers to our collaborators [including Rikard Blunck, University of Montreal, Canada] who immediately applied them and, to our surprise, both of these blockers significantly reduced TACAN-dependent currents,” he said.
A role for TACAN in nociceptors
The authors next asked whether TACAN underlies some of the mechanically induced currents observed in nociceptors. For these experiments, Beaulieu-Laroche and colleagues cultured DRG neurons from mice in which nociceptors could be visualized through the expression of a fluorescent protein. These neurons were then transfected with small interfering RNA (siRNA) expressing a fluorescent tag. The siRNA was targeted either to TACAN or a non-targeting control.
Reducing the expression of TACAN specifically in nociceptors using the siRNA reduced mechanically induced currents, which was accompanied by a reduction in the number of patches with active mechanically sensitive ion channels. And addition of GsMTx4 reversibly blocked mechanically induced currents.
“It was striking and assuring to see such clear differences between the TACAN knockdown and wild-type nociceptors as I was blinded throughout the experiments and the analysis,” said Beaulieu-Laroche.
Additional experiments would show that TACAN regulated ultra-slow adapting currents in DRG neurons.
What happens in vivo?
Was TACAN necessary for the detection of painful mechanical stimuli in vivo? Next turning to that question, the authors created a nociceptor-specific, inducible TACAN knockout mouse, since global knockout of TACAN is embryonically lethal. The group measured behavioral responses to hindpaw stimulation with mechanical stimuli of increasing intensity.
The investigators first examined the effect of TACAN knockout in Mrgprd-containing neurons. In control mice, mechanical stimulation (using von Frey filaments) with increasing stimulus strength increased the frequency of paw withdrawal reflexes until the intensity exceeded 1.0-1.4 grams. After that, these behavioral responses decreased in favor of nocifensive responses (withdrawal followed by licking of the hindpaw).
Meanwhile, the conditional TACAN knockout animals continued to display reflex behaviors but failed to show nocifensive behaviors despite the increasing stimulus intensity. This suggested that TACAN played a role in mechanosensation in the Mrgprd-containing, non-peptidergic nociceptor population.
“It is interesting to note that this data does not match previous studies where genetic ablation of Mrgprd-expressing neurons in adulthood leads to reduced withdrawal response frequency in the 1.0-1.4 gram range, a phenotype not seen at this force range in mice with conditional knockout of TACAN. This suggests that the Mrgprd neuron population might have another mechanically sensitive ion channel,” according to Ma (Cavanaugh et al., 2009).
In other experiments, the authors confirmed these findings by using short hairpin RNA (shRNA) to knock down TACAN in a broader population of DRG neurons that included mechanoreceptors. Here, too, they found that reducing TACAN expression affected only nocifensive behavioral responses and not withdrawal reflex responses.
Lastly, to address the possibility that the shRNA had only partially knocked down TACAN expression, potentially complicating the interpretation of their earlier findings, the group knocked TACAN down in all neurons. Confirming the previous two behavioral experiments, this caused a deficit only in nocifensive behavioral responses to mechanical stimuli, while leaving the withdrawal reflex intact.
“These results were really interesting because if we would have simply looked at the withdrawal responses, we would have not seen much,” according to Christin. “The difference really only became apparent when we looked at the nocifensive responses. Moreover, the consistency in these behavioral studies was really surprising to us. We actually had multiple people blinded to the genotype who tested these mice to make sure the phenotype was real.”
Importantly, the authors found that this loss of nocifensive behaviors was specific to mechanical stimuli because the animals continued to show normal responses to painful thermal stimuli. They also observed normal responses to pinprick, which is a model of intense mechanical stimulation that relies on peptidergic Aδ nociceptors (Arcourt et al., 2017PRF related news). This might suggest that Aδ nociceptors express another mechanically sensitive ion channel other than TACAN.
“Overall, the authors present a very compelling story with a very interesting behavioral phenotype where the TACAN knockout mice no longer sense punctate pressure-evoked pain. But it also raises more questions – for example, are different mechanically sensitive ion channels responsible for sensing different mechanical modes such as punctate pressure versus pinprick, pinch, or stretch – and confirms that we as a field have a lot more work to do in order to understand how we sense mechanical pain,” said Ma.
Get ready for more….
The study authors hope their work paves a path forward for other pain investigators.
“We hope that these findings help the field understand how pathological allodynia develops by offering researchers a new player in mechanical sensation of pain that they can investigate in the future,” said Christin.
In terms of their own future research, Sharif-Naeini’s laboratory is now investigating how TACAN’s responses to mechanical stimuli might change in response to injury.
“A channel does not need to be expressed at high levels in order to be physiologically relevant. If the nociceptor’s action potential threshold is lower due to an injury, even a low number of channels could cause an action potential,” said Sharif-Naeini.
“One of our previous studies on osteoarthritis pain has shown a leftward shift in the activation threshold of mechanically sensitive ion channels,” he continued (He et al., 2017). “Therefore, we want to investigate whether TACAN-dependent currents also exhibit this shift in activation threshold. Stay tuned for work from our lab.”
Francie Moehring is a freelance writer based in Milwaukee, US.