Transient receptor potential ankyrin 1 (TRPA1) is an ion channel expressed by many primary afferent sensory neurons. Allyl isothiocyanate (AITC), a pungent chemical that gives wasabi and mustard oil their powerful taste, is a well-known activator of the channel, as are substances produced by many other plants. In addition, a host of animals produce chemical irritants in their venoms that activate TRP channels in order to ward off predators. Now, a newly discovered and unique toxin from the venom of a scorpion is further illuminating the workings of TRPA1.
Research led by David Julius, University of California, San Francisco, US, along with first author John Lin King, a graduate student in neuroscience, and colleagues now identifies a peptide toxin from the venom of the Australian black rock scorpion (Urodacus manicatus) that modulates TRPA1 function. It does so by penetrating the plasma membrane and binding to the channel intracellularly, to prolong channel opening. Interestingly, this toxin elicits pain hypersensitivity without the typical accompanying neurogenic inflammation seen with other TRPA1 activators.
“[T]he work done by [the researchers] in identifying a new natural ligand of TRPA1 that stabilizes a unique channel conformation offers scientists both a new tool to probe TRPA1 receptor function and potential strategies for the future development of novel analgesics,” wrote Dustin Green and Xinzhong Dong, Johns Hopkins University School of Medicine, Baltimore, US, in an accompanying preview.
The research and preview were published September 5, 2019, in Cell.
Screening for a toxin that targets TRPA1
Previous work from the Julius laboratory identified the transient receptor potential vanilloid 1 (TRPV1) ion channel as the receptor target of an earth tiger tarantula toxin. The group also pinpointed acid-sensing ion channel 1 (ASIC1) as the receptor target of a coral snake toxin.
“We have a longstanding interest in reverse engineering the pain pathway using venoms, both peptides and small molecules, that plants and animals use to subdue their prey or ward off predators, and thus we were curious if other TRP channel toxins existed,” said Lin King.
Building upon this earlier work, the researchers carried out an unbiased venom library screen for toxins that would act on TRPA1 specifically. They expressed TRPA1 in human embryonic kidney 293 (HEK293) cells and used high-throughput calcium imaging to screen a large library of invertebrate venoms. Because many venoms contain components that can break down the cell membrane, the authors depleted the venoms of those components as a way to avoid false positive results. Using this strategy, the authors identified the venom of the Australian black rock scorpion as containing a TRPA1 activator.
Using liquid chromatography and mass spectrometry, the authors went on to identify a single active peptide and its primary amino acid sequence from the venom. Then they synthesized an identical peptide with the same activity as the native peptide. They put this synthetic peptide to use for the rest of their experiments, calling it the “wasabi receptor toxin” (WaTx).
Turning next to electrophysiological experiments, the researchers made whole cell recordings of HEK293 cells expressing human TRPA1 and discovered that WaTx elicited robust TRPA1 activation. Furthermore, a TRPA1 channel inhibitor completely prevented these TRPA1 currents, and neurons from animals missing TRPA1 did not respond to WaTx, whereas wild-type neurons did.
These and other experiments showed that the toxin could strongly activate TRPA1, and do so directly and selectively.
Getting inside
Classic TRPA1 agonists are smaller than WaTx, and also are electrophilic (“electron loving”). But since WaTx is a relatively large molecule and appeared to be non-electrophilic, this could mean that the toxin acts differently on the channel than typical TRPA1 activators.
To test that idea, the authors made patch clamp recordings, starting with an on-cell patch configuration (where the cell stays attached to the patch pipette, forming a tight seal). They saw that, before rupturing the membrane to gain access to the inside of the cell, WaTx evoked currents in single channels. This meant that the toxin was either getting through the cell membrane to reach channels under the patch pipette, or perhaps promoted the release of a signaling molecule in the cell cytoplasm that in turn activated TRPA1.
To learn which of those scenarios was at play, Lin King and colleagues next performed patch clamp recordings using inside-out and outside-out techniques. These are patch clamp configurations that give the toxin access to the intracellular site of TRPA1 in the former instance, or to the extracellular site in the latter case.
Also, to visually confirm that WaTx could cross the membrane, the authors used a fluorescence technique to demonstrate that WaTx readily crossed into liposomes (artificial vesicles similar to actual cell membranes).
“To our surprise, WaTx was able to evoke TRPA1 currents in either patch configuration. Furthermore, not only can it penetrate the membrane, but it needs to get across the membrane in order to reach the binding site the toxin is acting on,” said Lin King.
From rat snake to human, from human to rat snake
The investigators still did not know exactly where WaTx was acting. To learn more, the group took advantage of the fact that TRPA1 has evolved to serve different sensory functions in different species.
It turned out that TRPA1 from mammals was activated by WaTx, but TRPA1 from more distantly related species such as rat snakes, fish, flies, and worms was insensitive to WaTx. So Lin King and colleagues next used a so-called chimeric receptor approach. Here, they transferred part of TRPA1 from one species to the TRPA1 of another species in a dish. This would allow them to learn more about WaTx’s sensitivity for the ion channel.
Tellingly, when they transferred the C-terminal TRP domain from rat snake to human TRPA1, the latter became insensitive to WaTx but was still activated by AITC. This suggested that this particular region of the channel was critical for WaTx sensitivity for TRPA1.
But rat snake TRPA1 containing the human TRP domain was still insensitive to the toxin. This meant that this domain was necessary but not sufficient for WaTx’s sensitivity for the channel.
The C-terminal domain of TRPA1 interacts with the N-terminal domain of the channel, which is a region rich in cysteine amino acid residues; where this interaction takes place is known as the “allosteric nexus” that is required for activation of TRPA1 by electrophilic agonists. When the authors transferred this N-terminal domain from the human to the rat snake TRPA1, the rat snake TRPA1 chimera became sensitive to WaTx. This revealed that WaTx also acts at the allosteric nexus, just as electrophilic agonists do. Finally, amino acid substitution experiments showed that a single proline residue within the cysteine-rich linker region was essential for WaTx sensitivity for TRPA1.
Open for business
With its activity at the allosteric nexus, WaTx held something in common with its electrophilic cousins. So was there anything unique about this particular toxin in terms of how it activated the channel? It was to this question that the researchers next turned.
They saw that, compared to AITC, WaTx kept TRPA1 open longer, but did not shorten the time it took to open the channel. This suggested that WaTx stabilizes TRPA1 in its open state, rather than directly opens the channel.
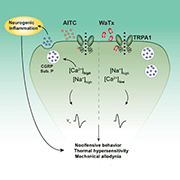
Further, while AITC activation of TRPA1 led to a notable preference of the channel for divalent cations like calcium, WaTx activation largely abolished that preference. This suggested that TRPA1 takes on a distinct open configuration when WaTx is bound to it versus electrophilic irritants.
Pain hypersensitivity but no inflammation
The group next examined the pain-related behavioral effects of WaTx, comparing it to the effects of AITC. Subcutaneous injection of WaTx or AITC into the hind paw of mice led to acute pain-related behaviors in wild-type animals, which was absent in TRPA1 knockouts.
Furthermore, like AITC, WaTx also led to expression of c-Fos, an indirect measure of neuronal activity, in superficial laminae of the spinal cord dorsal horn, and again only in wild-type animals and not in TRPA1 knockouts. This showed that WaTx engaged central nociceptive pathways.
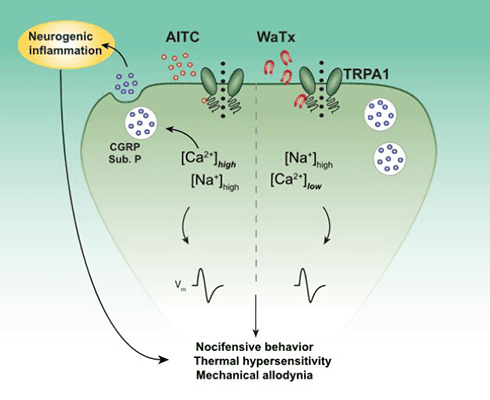
But then a crucial difference emerged. AITC also elicits hind paw swelling and increased vascular permeability, both being hallmarks of neurogenic inflammation that involves the release of neuropeptides such as calcitonin gene-related peptide (CGRP) and substance P; neurogenic inflammation is thought to be a key driver in the establishment and maintenance of chronic pain states. So the authors were excited to find that WaTx injection did not produce those effects, suggesting that WaTx does not cause neuropeptide release.
In confirmation of that idea, experiments using cultured sensory neurons under physiological calcium concentrations showed that AITC, but not WaTx, caused the release of CGRP. The authors suspected that this was because WaTx lowers the permeability of TRPA1 to calcium; high levels of calcium influx are necessary for neuropeptide release, and indeed the group showed that calcium influx through electrophile-activated TRPA1 was necessary and sufficient to elicit proinflammatory neuropeptide release. And, lo and behold, when looking at CGRP release in the setting of high extracellular calcium, they saw that WaTx was now able to bring about the release of CGRP.
“[T]he reason that this peptide’s function is restricted to inducing only non-inflammatory pain is unknown and highly intriguing. Has evolution deemed the helical hairpin design and low calcium permeability more energetically favorable for scorpions versus those proinflammatory peptides found in snake and tarantula venoms?” Green and Dong asked in their preview.
With regard to other evoked behaviors, subcutaneous WaTx resulted in strong and long-lasting mechanical and thermal hypersensitivity, just as AITC did.
In a final experiment, the authors took advantage of the fact that most TRPA1-expressing neurons also express TRPV1. So they ablated TRPV1-expressing central terminals by injecting capsaicin into the spinal canal, leaving peripheral terminals of TRPA1-expressing neurons intact.
In those ablation experiments, AITC produced hind paw swelling and mechanical hypersensitivity but not acute painlike behaviors. On the other hand, while WaTx also did not affect acute painlike behaviors, the animals showed no mechanical hypersensitivity, suggesting that the toxin primarily acts via a central mechanism to evoke pain.
In the end, “We hope that our work is pointing the way towards the molecular features of this critically important receptor involved in acute pain and instigation of chronic pain,” according to Julius.
Francie Moehring is a freelance writer based in Milwaukee, US.