Lysozyme, a bacteria-gobbling enzyme commonly known for its role in fighting infections as part of the innate immune system, may serve a previously unrecognized role in facilitating chronic pain after nerve injury. A new study from Saurabh Yadav and Avadhesha Surolia, Indian Institute of Science, Bangalore, India, shows that nerve injury enhances lysozyme expression, which increases the spontaneous activity of spinal cord dorsal horn neurons to produce painlike behaviors in rodents. The authors also identify the cellular protein annexin A2 and the innate immune receptor TLR4 as key components of this lysozyme-pain pathway.
“This is a very interesting and novel concept that the antibacterial enzyme lysozyme, which is a prominent innate immune enzyme, could also be expressed by neurons and contribute to neuropathic pain. Lysozyme is expressed during inflammation to help protect us against infections, and now having it coupled to pain is an interesting idea and identifies a potential new target for treatment,” said Isaac Chiu, Harvard Medical School, Boston, US, who studies interactions between the nervous and immune systems in the context of bacterial infections but was not involved in the study.
The new research appeared August 7, 2019, in Science Translational Medicine.
Lysozyme remains employed during pain, even without a bacterial-fighting job to do
As part of its bacteria-destroying (bacteriolytic) functions, lysozyme causes the release of substances such as peptidoglycans and lipoproteins that work downstream of the enzyme to activate Toll-like receptors (TLRs) and spur release of proinflammatory cytokines. These cytokines can sensitize nociceptors to produce the pain commonly associated with various types of bacterial infections. However, in the case of nerve injury, while inflammation is often present, bacteria are not, and therefore whether lysozyme played a part in neuropathic pain was unclear.
After a little digging into the pain research literature, first author Yadav said that some initial evidence for an association between lysozyme and neuropathic pain began to emerge.
“By examining databases of global gene expression changes in the context of neuropathic pain, I found multiple examples of lysozyme gene changes, which was quite interesting, as there was little or no information about its role in the context of pain. You expect lysozyme expression to change during an infection or during innate immune signaling, but the changes in the nervous system were intriguing to me,” he told PRF.
Yadav discussed the findings with Surolia, who had an interest in identifying new analgesic targets. “We have been studying some molecular inhibitors for the treatment of neuropathic pain, and when we discovered the upregulation of lysozyme, we decided to investigate the causal relationship between lysozyme overexpression and neuropathic pain,” Surolia said.
To begin, the researchers confirmed the upregulation of lysozyme in a neuropathic pain model. In particular, rats that underwent partial sciatic nerve ligation (PSL), an experimental model of neuropathic pain, showed upregulation of the enzyme in dorsal root ganglia (DRG) neurons, with accompanying persistent thermal and mechanical hypersensitivity of the hind paw, for up to two weeks, compared to sham control animals. Interestingly, Yadav and Surolia also found increased lysozyme expression in human spinal cord autopsy tissue from patients with spinal cord injury.
But the source of lysozyme was unclear, since infiltrating macrophages can also contribute to increased expression of the enzyme following nerve injury. Using intrathecal minocycline to inhibit the activation of macrophages and glial cells in PSL rats, the investigators saw no decrease in DRG lysozyme expression post-nerve injury, suggesting a neuronal origin of lysozyme in this circumstance. Further evidence for this was an approximately fourfold increase in lysozyme expression in DRG neurons but not in glial cells following PSL.
Both thermal and mechanical hind paw hypersensitivity following PSL were reduced by intrathecal injection of either of two different inhibitors of lysozyme, including chitobiose and bromophenol blue. Intrathecal small interfering RNA (siRNA) directed against lysozyme also substantially reduced hind paw hypersensitivity. These findings provided evidence for a role of lysozyme in painlike behaviors in rats after nerve injury.
Exactly what causes increased lysozyme expression following nerve injury in the first place is unclear. According to Chiu, one possible explanation, though a speculative one, could be the resident bacteria in the gut, known as the microbiome.
“Normally, the microbiome is separate from neurons via the intact gut barrier, but in the case of nerve injury the gut barrier may become compromised, and microbial components could act on neurons directly or on immune cells to induce lysozyme or other gene expression changes,” Chiu said.
Delayed sensitivity
If lysozyme is upregulated after nerve injury and blocking it can ameliorate painlike behaviors, what effect would external lysozyme administration have on naïve (non-nerve injured) animals? To address this question, Yadav and Surolia injected lysozyme directly into the spinal cord of healthy rats and saw hind paw thermal and mechanical hypersensitivity beginning 30 minutes after injection and lasting for about 90 minutes.
The researchers also wanted to understand if lysozyme’s bacteriolytic activity contributed to its pain-promoting actions. So they compared the effects of intact, whole lysozyme versus the effects of only the N-terminal peptide region of the enzyme, which is responsible for lysozyme’s bacteria-killing effects but not its broader enzymatic effects. Intact lysozyme produced painlike behaviors in otherwise healthy rats, while the N-terminal fragment had no impact. This indicated that the bacteriolytic activity of lysozyme was not required for the enzyme to evoke pain.
Lysozyme also produced spontaneous neuronal excitability in spinal cord slices from healthy animals. In particular, both A-delta fibers and C-fibers showed increased spontaneous firing after lysozyme application. Interestingly, the effects on both fiber classes were slow in onset, taking about 30 minutes to emerge.
“The fact that we see a delay in both our behavioral and electrophysiology experiments suggests that lysozyme is not acting directly on ion channels, because if that were the case, we would expect to see almost instantaneous effects after lysozyme application,” explained Yadav.
A novel pathway
The relatively slow onset of painlike behavior and neuronal excitability led the team to search for molecular targets of lysozyme that might underlie these effects. Using a mass spectrometry assay, they showed that annexin A2 interacted with lysozyme in rat DRG neurons. Existing in two forms, one found mostly in the cell cytoplasm and the other at the cell surface, annexin A2 is a protein involved in a host of cellular processes ranging from trafficking protein to the cell membrane, to cell proliferation and division. The researchers further determined that the cell surface form of annexin interacted the most strongly with lysozyme, compared to the cytoplasmic form.
Inhibiting cell surface annexin A2 via a targeted siRNA in healthy rats three days before administering lysozyme substantially improved thermal and mechanical allodynia. That suggested the existence of a lysozyme-annexin A2 signaling pathway underlying the pain-promoting effects of lysozyme in rodents.
The researchers knew that annexin A2 can activate TLR4, a key component of the innate immune system. They also knew that TLR4 had previously been shown to play a role in chronic pain (Lacagnina et al., 2018). So to test whether TLR4 was part of the signaling pathway, the investigators pretreated uninjured rats with a TLR4 inhibitor prior to lysozyme administration, which reduced the heightened pain response. Giving lysozyme to mice with defective TLR4 receptors also did not produce any painlike behaviors, compared to TLR2 knockout mice.
“Lysozyme recruits annexin A2 to activate TLR4 and essentially to do its bidding, as by itself it cannot bind to TLR4. The lysozyme-annexin A2 complex is crucial, and this partnership is required for lysozyme-mediated neuropathic pain,” Surolia told PRF.
TLR4 is expressed on both neurons and microglia. But while lipopolysaccharide (LPS, the major component of the outer membrane of certain bacteria) is known to activate TLR4 expressed on microglia, it was unclear whether lysozyme activated neuronal or glial TLR4. To address this, the researchers gave an inhibitor of both MD2 and CD14 (two proteins required for LPS binding to and activation of TLR4) intrathecally to naïve rats, followed by lysozyme. They discovered that lysozyme produced pain responses in these animals, while LPS injection, used as a control, had only minor effects on pain in rats pretreated with the inhibitor. This demonstrated that glial TLR4 was not required to facilitate neuropathic pain, suggesting that perhaps neuronal TLR4 was the culprit.
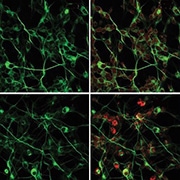
![Lysozyme stimulates neuronal excitation in human primary neurons. Immunofluorescence micrograph analysis of C-FOS expression [an indirect measure of neuronal activity] in human primary neurons upon treatments with lysozyme, Cu CPT-22 (TLR2 inhibitor) and TAK-242 (TLR4 inhibitor). Differentiated primary neuronal cells were marked with β-III-tubulin (green). Image and credit from Yadav S, Surolia A. Lysozyme elicits pain during nerve injury by neuronal Toll-like receptor 4 activation and has therapeutic potential in neuropathic pain. Sci Transl Med. 2019 Aug 7; 11(504). Reprinted with permission from AAAS.](https://www.iasp-pain.org/wp-content/uploads/2023/02/LysozymeINLINE.png)
Finally, experiments in human spinal cord autopsy tissue confirmed the lysozyme-TLR4 interaction, suggesting the possibility that lysozyme could facilitate nerve injury-associated pain in people. Work using human primary neurons also showed that lysozyme increased activity of these cells.
Although the evidence for a role of TLR4 seems compelling, Chiu said the use of TLR4 knockout mice might provide more definitive evidence; the current study used mice with a strain background that had a mutated receptor (known as C3H/HeJ mice).
“The C3H/HeJ mice certainly have a mutated TLR4 receptor that interferes with its ability to interact with LPS, but it is not clear whether the mutation also interferes with TLR4 interaction with annexin. Maybe a pure wild-type versus a TLR4 gene knockout strain would have answered this question more conclusively.”
As for future work, Surolia is particularly interested in the delay seen from the time of lysozyme administration until the behavioral and neuronal effects appear.
“We don’t know what is happening during that time, so now we are going to probe the role that excitatory neurotransmitters may play. We have shown that lysozyme promotes excitatory signaling to excite neurons, but we don’t know as of yet how it does so or what neurotransmitters may be involved.”
Dara Bree is a postdoctoral fellow at Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, US.