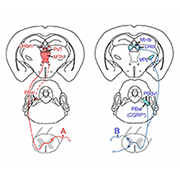
The response to a painful stimulus generally consists of two phases: a quick withdrawal from the stimulus, followed by behaviors such as rubbing or licking of the injured area to help ease the suffering. While these responses are well recognized by pain researchers, whether the same neuronal circuits underlie them remains poorly understood.
Now, a new mouse study led by Qiufu Ma, Dana-Farber Cancer Institute and Harvard Medical School, Boston, US, identifies spinal neurons marked by the expression of tachykinin (TAC1) during development as responsible for what the researchers describe as “coping” behaviors associated with sustained pain, including rubbing and licking of the hind paw, over a period of several minutes.
Further, selective ablation of the TAC1-lineage neurons resulted in a loss of these behaviors, as well as a loss of conditioned place aversion evoked by pinch, but without affecting reflexive defensive reactions to external threats. Conversely, optogenetic activation of the neurons was sufficient to produce soothing behaviors in the animals.
“This study targets spinal TAC1-lineage neurons which include projection neurons and a sizable number of interneurons,” said Rebecca Seal, University of Pittsburgh, US, who was not involved in the research. “One of the difficulties in trying to figure out the neural circuitry for pain is finding ways to specifically manipulate functional subsets of neurons. In this case, projection neurons were the intended target, and when activated, induced aversive behaviors. Nevertheless, the study did not rule out a contribution by TAC1-lineage interneurons,” Seal continued.
Whether the behaviors examined in the study represent “coping” has become a matter of debate, and the relevance of the findings to chronic pain also awaits further study. Nevertheless, what Ma hopes most for his new paper is that it spurs more discussion about the measurement of acute and chronic pain.
It’s time to “revisit current acute pain measurement in animal studies, and it’s urgent for future studies to distinguish sensitized reflexes versus sensitized pain under chronic pain conditions,” Ma said.
The research was published online December 10, 2018, in Nature.
Different responses, different pathways
Ma spent the early years of his career characterizing the molecular features of excitatory and inhibitory neurons in the dorsal horn. Collaborating with Martyn Goulding, Salk Institute for Biological Studies, La Jolla, US, Ma and his colleagues employed intersectional genetic techniques to make several mouse lines that enable targeting of different spinal neuron populations. This included somatostatin-expressing neurons, which they showed were responsible for transmitting mechanical pain, along with neurons expressing TAC1.
“We previously identified somatostatin neurons as those that drive both reflexes and sustained pain-associated licking evoked by noxious mechanical stimuli under acute conditions, and that drive both punctate and dynamic mechanical allodynia following inflammation or nerve injury,” Ma said (see related PRF story here). “We also reported that spinal VGLUT3-lineage neurons are required selectively to drive chronic dynamic allodynia, and the loss of this clinically more relevant form of neuropathic pain was not detected by von Frey filament assays [Cheng et al., 2017]. In this new study, we turned our attention to distinct spinal substrates and primary sensory neurons to see if they differentially drive reflexive defensive reactions to external threats versus behaviors associated with sustained pain” that soothe suffering, explained Ma.
To address this question, co-first authors Tianwen Huang, Shing-Hong Lin, and colleagues first examined the expression of TAC1 in spinal neurons and their projections to brain regions that process the unpleasantness of noxious stimuli. Focusing on the thalamus, the team discovered that these neurons selectively innervated areas of the medial thalamic pathway, which processes the unpleasantness resulting from sustained, intense painful stimuli. TAC1-lineage neurons also innervated the superior lateral parabrachial nuclei that may in turn send projections to the medial thalamic nuclei.
Ablating spinal TAC1-lineage neurons in mice had little effect on standard paw withdrawal thresholds to mechanical or thermal stimulation. This suggested that these neurons were largely dispensable for reflexive, defensive responses to external threats. However, TAC1-lineage neurons appeared necessary for behaviors such as persistent paw licking that may serve to soothe sustained pain. Compared with non-ablated control mice, TAC1-ablated mice showed substantially reduced episodes of hind paw licking in several models of sustained pain, including the hotplate test, mustard oil administration (which activates TRPA1 receptors), and hind paw burn injury.
Interestingly, TRPV1 activation via hind paw capsaicin injection produced robust licking episodes in both TAC1-ablated and control mice, suggesting the presence of pain pathways regulating sustained pain that are independent of TAC1 signaling.
Neurons in a pinch
To investigate behaviors produced by intense and sustained mechanical pain, the researchers, working with Robert LaMotte and Nathalie Malewicz at Yale University School of Medicine, New Haven, US, employed a translational approach using an alligator clip to pinch the skin of both human subjects and mice. The former were asked to report the pain felt upon clip application, and they reported a peak maintained pain intensity within 15 seconds, which corresponded with the time course of peak licking episodes observed in wild-type mice.
“It was very interesting to see that the sustained pain rating in humans following the pinch almost temporally matched the sustained ongoing licking behavior in mice, suggesting that skin pinch-evoked licking could be used to measure sustained mechanical pain in animal studies,” said Ma.
The persistent licking behavior was significantly reduced in TAC1-ablated mice during the one-minute period in which the clip was in place. Increased c-Fos expression, a surrogate marker of neuronal activity, in both the spinal cord and regions of the medial thalamic pathway accompanied the increased licking behavior in control mice, while c-Fos expression was substantially lower in TAC1-ablated mice.
“The fact that von Frey thresholds measured in the absence of injury were not altered in the TAC1-ablated mice suggests that the TAC1-lineage spinal neurons likely get obligatorily recruited only when you have a more sustained noxious stimulus such as pinching,” explained Seal.
Next, using a conditioned place aversion (CPA) assay, the researchers also demonstrated that control mice learned to avoid a pinch-paired compartment, but TAC1-ablated mice did not display this conditioned response. The researchers then asked whether activating the TAC1-lineage neurons would be sufficient to produce CPA in the absence of any noxious stimulus. To address this question, they employed optogenetic techniques to selectively activate TAC1-expressing neurons in the medial thalamic pathway. The mice avoided a light-stimulated chamber, suggesting that activation of these ascending projection neurons was sufficient to drive CPA.
Is it coping?
The use of the term “coping” to describe the behaviors of the mice in response to sustained pain is problematic to some, but Ma acknowledges this.
“During a recent workshop on pain models at the NIH [National Institutes of Health] some researchers told me that they had issues with the use of the word ‘coping’ and whether they thought it was correct, while other researchers were fine with it,” Ma told PRF. “In this study we used ‘coping’ to describe an ongoing behavior towards an injured area that tries to reduce the suffering caused by inescapable sustained pain. We struggled to find a better word to describe these behaviors,” according to Ma.
Regardless, he hopes his work will spark further conversation about the best way to assess such animal behaviors evoked by stimuli that also produce sustained pain in humans. “Current approaches that use withdrawal reflexes or defensive reactions in animals are not ideal, as they likely reflect innate responses to external threats or, at best, the first sharp pain percepts and not the suffering components of sustained pain,” Ma told PRF.
Looking to the periphery
Ma and colleagues believe their findings show, at the spinal level, a functional segregation of neurons that process reflexive, defensive responses from those that regulate soothing behaviors in the presence of sustained pain. Taking this a step further, the authors were keen to investigate whether this segregation also existed in the periphery, particularly at the level of primary afferent fibers. They focused on two mostly non-overlapping classes of nociceptors marked by the expression of MRGPRD or TRPV1, which innervate the skin epidermis and the whole body, respectively.
They chemically ablated either adult MRGPRD neurons or the central terminals of TRPV1 neurons in mice. The team discovered that withdrawal thresholds to mechanical stimulation were increased in MRGPRD-ablated mice while pinch-evoked licking responses were unaltered, compared to control, non-ablated mice. Conversely, TRPV1 ablation did not affect mechanical withdrawal thresholds but caused a reduction in the number of licking episodes evoked by skin pinching or skin burn injury. This provided evidence of a functional segregation of peripheral nociceptors for processing different aspects of the response to a painful stimulus. Furthermore, c-Fos expression in TAC1-lineage spinal neurons following skin pinch was reduced in TRPV1-ablated mice, suggesting a functional connection between those spinal neurons and TRPV1 nociceptors.
Interestingly, the researchers say that by examining more than only reflex assays, their results cast doubt on a previously held conclusion that MRGPRD nociceptors are required to drive sustained acute mechanical pain (Cavanaugh et al., 2009), since they showed in their new study that it was TRPV1 neurons that were critical for this.
Whether or not there is an interdependent relationship between peripheral TRPV1 nociceptors and spinal TAC1-lineage neurons remains to be addressed. “Based on the findings, you would think that there may be a specific relationship between activation of TAC1-lineage neurons by TRPV1 primary sensory neurons and not by MRGPRD neurons. Taking into consideration the TRPV1 ablation results, however, it is a bit curious why capsaicin-induced licking was not reduced in the TAC1-lineage ablated mice,” according to Seal.
As for future work, Ma wants to see whether the results will apply to chronic pain as well, something that also remains uncertain.
“This study used acute pain models and investigating if the functional segregation of neuronal pathways exists in chronic pain will be important,” Ma said.
Dara Bree is a postdoctoral fellow at Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, US.
Image credit: Huang et al., 2018, with permission from Springer Nature.